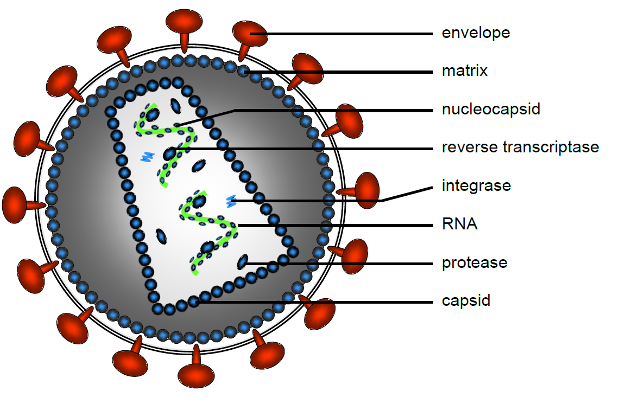
Schematic representation of the molecules making up a retrovirus, Courtesy of Karin Pike-Overzet
Professor Staal’s Molecular Stem Cells Biology laboratory at the Leiden Universtity Medical Center has received a grant from ZonMW, the Dutch health care research funding organisation, to develop a clinical gene therapy treatment for RAG1 deficiency.
During this project, which consists of a preclinical phase and a pharmaceutical phase, a lentiviral vector will be optimized for transfer of the a codon optimized RAG1 gene into hematopoietic stem cells. During the pharmaceutical phase, this vector will be produced and finally tested before a clinical trial is started. We have entered a preclinical, pharmaceutical and clinical program to develop gene therapy for RAG1-SCID. This effort is supported by ZonMW, the Netherlands organization for health research.
Nederlandse samenvatting / Summary in Dutch
Genetisch gemodificeerde stamcellen voor genezing van een ernstige gecombineerde afweerstoornis. Bij SCID (ernstige gecombineerde afweerstoornis) zijn T lymfocyten, de belangrijkste cellen van het specifieke afweersysteem, afwezig. Kinderen met deze aandoening kunnen behandeld worden met beenmergtransplantatie, maar zonder geschikte donor (wat in het merendeel van de gevallen zo is) overlijden de kinderen aan ernstige infecties. Gentherapie kan dan een oplossing bieden. De huidige subsidie richt zich op een erg lastig gen voor de gentherapie bij SCID, namelijk het RAG1 gen, dat direct bij de ontwikkeling van lymfocyten is betrokken. Wij gaan stamcellen van de patiënt modificeren zodat er met een kreupel virus weer een goede kopie van het RAG1 gen ingebracht wordt. Inmiddels hebben wij het juiste kreupele virus, de zogenaamde vector, gemaakt en kunnen laten zien dat in het werkzaam is muizen model van RAG-SCID. Deze vector wordt nu op klinische schaal geproduceerd door een faciliteit in Engeland. Ook is weesgeneesmiddel status verkregen bij de EMA en is begonnen aan het schrijven van het uitgebreide klinische protocol. Uiteindelijk mondt het project uit in een klinische studie.
RAG1 gene therapy project summary
Severe combined immunodeficiency (SCID) comprises a group of rare diseases in which cells of the adaptive immune system are unable to develop. The phenotype of SCID depends on the underlying genetic defect. SCID patients with a mutation in the recombination activation gene 1 (RAG1) lack both T- and B lymphocytes and suffer from severe infections. Allogeneic hematopoietic stem cell (HSC) transplantation is a curative treatment for SCID; however, a suitable donor is rarely available. For two other types of SCID, X-SCID and ADA-SCID, gene therapy using gene corrected autologous HSC has been successfully developed, with ongoing modifications to improve long-term safety and efficacy.
Based on our preclinical work in mouse models, and the experience gained in the clinical trials targeting X-SCID and ADA-SCID, we now propose to develop a preclinical and pharmaceutical program leading to a clinical gene therapy trial for RAG1-SCID. To this end we have assembled an international consortium of basic scientists, clinicians, pharmacists and patient advocacy groups with ample experience in developing and conducting such a program. Halfway into the program, we have successfully generated a novel coRAG1 vector that fully restores the deficiency in RAG1 KO mice. The vector is currently being produced at GMP grade in England and the IMPD has largely been written. We hope to start clinical studies before the end of 2016.
Milestones achieved
- Four different RAG1 vectors have been produced at the Experimental Hematology department of Hannover Medical School and tested in Leiden
- One of these worked well and is efficacious in the RAG1 KO mouse model
- Lentiviral plasmids have been produced at Plasmid Factory (Bielefeld, Germany)
- Vector being produced at clinical and pharmaceutical grade at the Rayne Cell Therapy Suite at King’s College in London
- Orphan disease status was granted by EMA
- IMPD and clinical protocol are being written in close colaboration with the Interdivisional GMP Facility LUMC (IGFL) and pediatrics departments, together with the gene therapy team at UCL in London.
Gene Therapy for Immunodeficiencies
Current research is focused on the optimization and clinical translation of gene therapy of RAG1 deficiencies. This project brings together over 10 years of preclinical work and should result in application of lentiviral gene therapy of a codon optimized RAG1 in hematopoietic stem cells. In preclinical pipeline, we have also developed lentiviral gene therapy vectors for BTK deficiency and RAG2 deficiency.